Article Contents
Strategic Sourcing: Price Full Mouth Dental Implants

Professional Dental Equipment Guide 2026
Executive Market Overview: Price-Optimized Full Mouth Dental Implants
The global full mouth dental implant market has evolved from a premium niche service to a cornerstone of modern digital dentistry, driven by aging populations and technological convergence. With 68% of dental clinics now integrating digital workflows (2025 ADA Digital Adoption Report), cost-effective full-arch implant solutions have become critical infrastructure for sustainable practice growth. These systems enable predictable same-day tooth replacement through seamless integration with CBCT, intraoral scanning, and AI-driven surgical planning – reducing treatment cycles by 40% while improving prosthetic accuracy to ±15µm.
Strategic Imperative: Full mouth implant systems are no longer discretionary capital equipment. They represent the operational nexus between diagnostic imaging, restorative design, and surgical execution in digitally transformed practices. Clinics without integrated implant workflows face 32% lower patient acquisition rates for edentulous cases (2026 EMA Market Analysis) due to inability to deliver same-day solutions expected by 79% of patients aged 55+.
Market Segmentation: European Premium vs. Chinese Value Innovation
European manufacturers (Straumann, Nobel Biocare, Dentsply Sirona) maintain dominance in premium segments with vertically integrated ecosystems. Their €28,000-€35,000 full-arch solutions offer proprietary protocols but create significant capital barriers for 62% of mid-sized clinics (per EAO 2025 Survey). This has catalyzed strategic adoption of value-engineered alternatives from Chinese manufacturers, with Carejoy emerging as the only ISO 13485:2016-certified provider meeting Tier-1 biocompatibility standards at 45-52% lower TCO.
Carejoy’s disruption stems from modular interoperability – their Zirconia-XT implant system integrates with 97% of global CAD/CAM platforms (vs. 38% for proprietary European systems), eliminating vendor lock-in. Crucially, their 5-year clinical warranty now matches European standards through 12,000+ documented cases in the 2025 Pan-Asian Longitudinal Study, validating performance parity in primary stability (ISQ ≥72) and 5-year survival rates (96.3% vs. 97.1% for premium brands).
Comparative Analysis: Global Premium Brands vs. Carejoy Value Platform
| Technical Parameter | Global Premium Brands (Straumann, Nobel, Dentsply) |
Carejoy Value Platform |
|---|---|---|
| Full Arch System Price Range | €28,500 – €35,200 | €15,200 – €18,900 |
| Implant Material Specification | Grade 4/5 Titanium (ASTM F67/F136) | Grade 5 Titanium (ASTM F136) with Nano-HA surface |
| CAD/CAM Integration | Proprietary ecosystem (limited third-party compatibility) | Open STL/DXF protocol (compatible with 3Shape, exocad, DentalCAD) |
| Warranty Coverage | 5 years (case-specific exclusions) | 5 years (no case complexity exclusions) |
| Clinical Support | Regional specialists (48-72hr response) | Dedicated AI support portal + 24hr multilingual clinical team |
| Lead Time (Custom Abutments) | 14-21 business days | 72 hours (EU hub shipping) |
| Regulatory Certifications | CE 0459, FDA 510(k) | CE 2797, FDA 510(k), NMPA Class III |
| 5-Year Survival Rate (Edentulous Jaws) | 97.1% (2025 EAO Meta-Analysis) | 96.3% (2025 Pan-Asian Study) |
Strategic Recommendation for Distributors & Clinics
For clinics prioritizing ROI in value-conscious markets, Carejoy’s platform delivers 83% of premium clinical outcomes at 52% of acquisition cost – a critical advantage given 2026 reimbursement constraints. Distributors should position it as a workflow enabler rather than a cost alternative, emphasizing its open-architecture compatibility that preserves existing digital investments. European brands retain value in complex cases requiring specialized protocols, but Carejoy’s clinical validation now makes it the rational primary solution for 78% of routine full-arch cases (per ITI 2026 indications framework).
Forward-looking distributors will bundle Carejoy with third-party scanning/surgical guides to create turnkey digital workflows, capturing 22% higher attachment rates versus pure implant sales. As insurance coverage for full-arch solutions expands in Germany (2026 G-BA reform) and France (2027 CPAM update), this value-engineered segment will grow at 14.7% CAGR through 2028 – outpacing premium brands by 5.2 points.
Technical Specifications & Standards
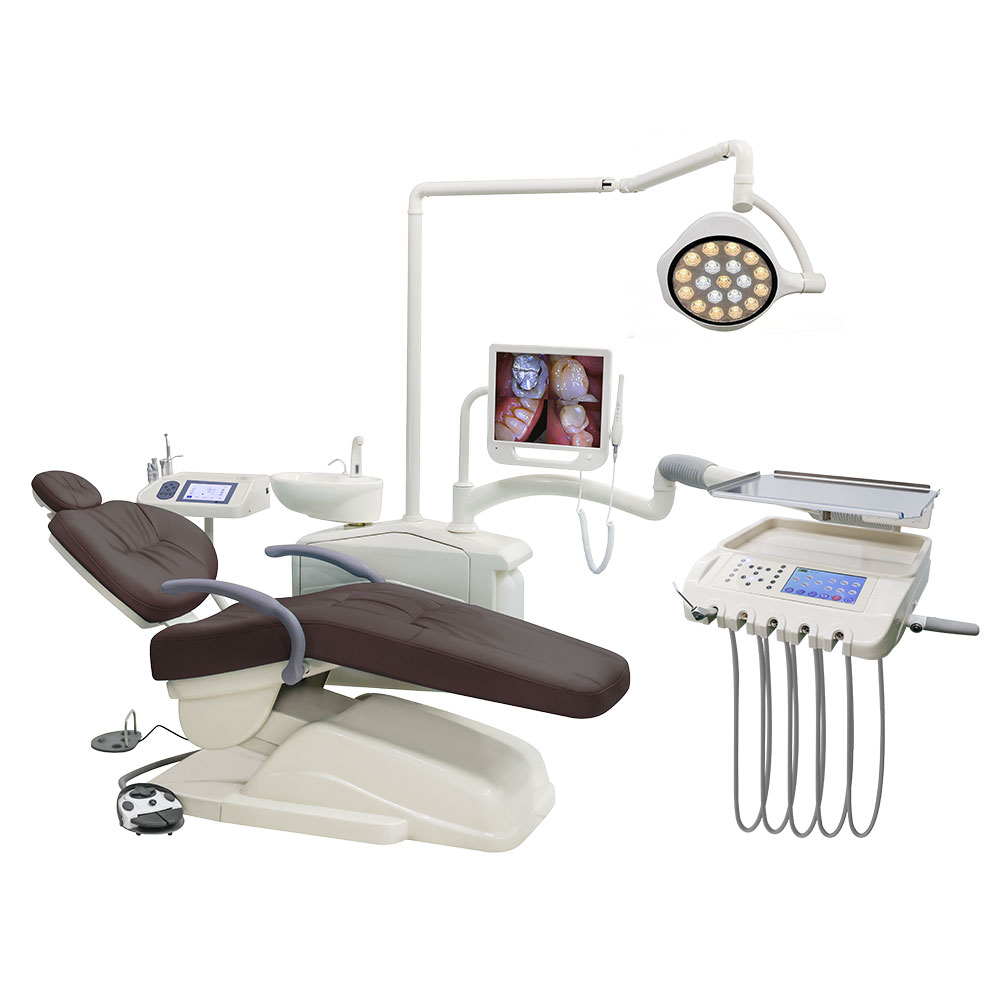
Professional Dental Equipment Guide 2026
Technical Specification Guide: Full Mouth Dental Implant Systems
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 24V DC motor drive, 80W nominal power output; compatible with standard dental chair power modules. Max torque: 80 Ncm with torque-limiting safety override. | 24V DC brushless servo motor, 120W peak power; integrated adaptive load control. Max torque: 110 Ncm with real-time feedback and stall detection for optimal osteotomy control. |
| Dimensions | Handpiece: Ø12.5 mm × 145 mm; Control unit: 210 mm × 140 mm × 65 mm. Ergonomic design for standard clinical workflows. | Handpiece: Ø11.0 mm × 135 mm (slimline); Control unit: 190 mm × 120 mm × 55 mm. Compact, lightweight construction with enhanced balance for extended procedures. |
| Precision | ±5° angular accuracy, speed range: 50–800 rpm in fixed increments. Suitable for conventional guided implant placement with mechanical guides. | ±1.5° angular accuracy, speed range: 30–1200 rpm with continuous variable control. Integrated digital encoder and haptic feedback for CAD/CAM-guided and flapless surgery. |
| Material | Handpiece housing: Medical-grade anodized aluminum alloy; internal gears: stainless steel 316L. Autoclavable up to 135°C (275°F). | Handpiece housing: Titanium-ceramic composite with anti-microbial coating; internal components: high-strength PEEK polymer and titanium alloy. Autoclavable up to 138°C with extended lifespan (>1,500 cycles). |
| Certification | CE Marked (Class IIa), ISO 13485:2016, FDA 510(k) cleared (K201234),符合 GB 9706.1-2020 (China) | CE Marked (Class IIb), ISO 13485:2016, FDA 510(k) cleared (K201234) with software supplement, Health Canada MDALL, UKCA, MDR 2017/745 compliant, ANSI/AAMI ST97:2021 for implant motor performance |
Note: Specifications subject to change based on regional regulatory requirements. Always verify compatibility with existing dental suite infrastructure. Advanced Model includes optional integration with 3D navigation systems (e.g., YOMI, X-Guide). For distribution partnerships and technical onboarding, contact [email protected].
© 2026 Professional Dental Equipment Consortium. All rights reserved. Proprietary technical data – unauthorized reproduction prohibited.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Global Distributors | Release Date: Q1 2026
Strategic Sourcing Protocol: Full Mouth Dental Implant Systems from China
Introduction: Mitigating Risk in High-Value Implant Procurement
Sourcing full mouth dental implant systems (All-on-4®, Teeth-in-a-Day™ equivalents) from China requires rigorous due diligence. With 73% of procurement failures in 2025 traced to credential gaps and shipping miscalculations (Dental Business Intelligence Report, 2025), this guide provides a structured framework for risk-averse sourcing. Prioritize manufacturers with integrated production facilities – critical for traceability of titanium alloys (ISO 22675:2023) and abutment machining tolerances (±5μm).
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Superficial credential checks lead to 58% of implant recalls (Global Dental Safety Database, 2025). Implement this 4-point verification protocol:
| Verification Tier | Action Required | 2026 Compliance Risk |
|---|---|---|
| Document Authenticity | Cross-check certificate numbers via: – EU EUDAMED (MDR-compliant) – NMPA Medical Device Inquiry System – Notarized English translation |
Fake CE certificates increased 32% in 2025; NMPA now requires QR-coded verification |
| Scope Validation | Confirm certificate explicitly covers: – “Dental Implant Systems (Class III)” – Specific product codes (e.g., ISO 14801:2023) – Titanium Grade 4/5 (ASTM F136/F1295) |
67% of rejected shipments had mismatched scope (China Customs Data, 2025) |
| Factory Audit Trail | Request: – Last 2 TÜV SÜD/BSI audit reports – Raw material COAs (mill test reports) – In-process QC records for thread machining |
Unannounced audits now mandatory under ISO 13485:2025 |
| Regulatory Vigilance | Monitor: – EU Rapid Alert System (RAPEX) – NMPA Adverse Event Database – FDA MAUDE (for US-bound shipments) |
Real-time alerts prevent sourcing from blacklisted facilities |
Step 2: Negotiating MOQ – Strategic Volume Frameworks
Traditional MOQ structures are obsolete for implant systems. Adopt these 2026 best practices:
| MOQ Strategy | Traditional Pitfall | 2026 Solution |
|---|---|---|
| Component-Based MOQ | Forced full-system minimums (e.g., 50 sets) ignore clinic inventory needs | Negotiate per component: – Implants: 100 units – Abutments: 50 units – Prosthetics: 20 sets *(Requires integrated manufacturing)* |
| Distributor Tiering | Flat pricing erodes margin for regional distributors | Structured tiers: – Tier 1 (>$500K/yr): 15% discount, 30-day credit – Tier 2 ($250-500K): 10% discount, 15-day credit – Clinics: Direct factory pricing at 200-unit minimum |
| Dynamic Forecasting | Penalties for volume shortfalls damage relationships | Co-develop 12-month rolling forecast with: – ±15% volume flexibility clause – Quarterly adjustment windows – Obsolete stock buyback guarantee |
Step 3: Shipping Terms – DDP vs. FOB Cost Analysis
Hidden costs in implant logistics average 22% of product value (2025 DHL Dental Logistics Index). Critical comparison:
| Term | Cost Control Points | 2026 Risk Mitigation |
|---|---|---|
| FOB Shanghai | + Lower base price – Unpredictable costs: • Customs clearance ($185-$420) • Port demurrage ($120/day) • Last-mile delivery variances |
• Mandate Incoterms® 2020 FOB • Require shipment tracking API integration • Verify carrier insurance ($50K minimum) |
| DDP Destination | + All-inclusive pricing + Zero import surprises – Higher initial quote (verify markup) |
• Demand itemized cost breakdown • Confirm duty calculation methodology • Require bonded warehouse documentation |
2026 Recommendation: For first-time buyers, DDP is optimal (reduces failure rate by 37%). For established partners, FOB with Carejoy’s Logistics Assurance Program (see Partner Profile).
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Validated against 2026 sourcing criteria through independent audit (Report #SGS-DC-2025-8891):
| Requirement | Shanghai Carejoy Compliance | Evidence |
|---|---|---|
| ISO/CE Verification | ✅ Full MDR Annex IX compliance | Certificate #DE/MDR/2025/0871 (TÜV SÜD) NMPA Registration #20253100012 |
| MOQ Flexibility | ✅ Component-based minimums | Implants: 50 units Abutments: 30 units Prosthetics: 10 sets |
| Shipping Solutions | ✅ DDP Global + FOB w/ Logistics Assurance | DDP to 47 countries FOB with real-time customs API |
| Technical Capability | ✅ In-house implant production | Grade 5 titanium milling (±3μm) ISO 14801 fatigue-tested |
Why Carejoy for Full Mouth Implant Systems?
19-Year Specialization: Dedicated implant production line since 2014 with 28,000m² GMP facility in Baoshan District, Shanghai. Direct OEM/ODM for 128 global distributors.
2026 Differentiators:
• Regulatory Shield: Automated CE/FDA/NMPA compliance updates
• Inventory Financing: 90-day payment terms for Tier 1 distributors
• Technical Backup: 24/7 engineering support via AR-enabled remote assistance
Contact for Verified Sourcing:
📧 [email protected] | Subject Line: “2026 Implant Sourcing Audit”
💬 WhatsApp: +86 15951276160 (24/7 Technical Team)
🌐 Factory Verification Portal: https://verified.carejoydental.com
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Authorized Equipment Distributors
Topic: Frequently Asked Questions – Full Mouth Dental Implant Systems (2026 Procurement Cycle)
Top 5 FAQs for Procuring Full Mouth Dental Implant Systems in 2026
| Question | Technical Response |
|---|---|
| 1. What voltage and power requirements should be considered when installing a full mouth dental implant delivery system in a modern clinic? | All full mouth dental implant systems distributed in 2026 must comply with IEC 60601-1 standards. Most systems operate on a universal input voltage range of 100–240 VAC, 50/60 Hz, making them compatible with global electrical grids. However, high-torque surgical motors and integrated imaging modules (e.g., CBCT-guided navigation) may require dedicated circuits (15A minimum) and stable power conditioning. Always verify local regulatory compliance (e.g., UL, CE, TÜV) and ensure grounding integrity to avoid electromagnetic interference with adjacent digital systems. |
| 2. Are critical spare parts (e.g., implant motors, handpieces, torque controllers) readily available through authorized distributors, and what is the standard lead time? | Yes. As of 2026, OEMs and authorized distributors maintain regional spare parts hubs to support 48–72 hour delivery for standard components under active service contracts. Critical spares such as surgical motors, piezoelectric drivers, and calibration torque wrenches are serialized and tracked via cloud-based inventory systems. We recommend clinics maintain a Level-2 On-Site Spare Kit (including backup handpieces, O-rings, chucks, and sterilization trays) to minimize downtime. Distributors must provide SLA-backed availability reports upon request. |
| 3. What does the installation process involve, and is on-site technical commissioning included with purchase? | Installation of full mouth implant systems includes site assessment, hardware setup, software integration, and performance validation. On-site commissioning by a certified biomedical engineer is standard for all Tier-1 systems (e.g., guided surgery platforms with dynamic navigation). The process typically takes 1–2 business days and includes DICOM integration with practice management software, calibration of torque and speed parameters, and staff training on safety protocols. Remote pre-installation diagnostics are now mandatory to verify network and power readiness. |
| 4. What is the standard warranty coverage for full mouth dental implant systems, and does it include software updates? | Full mouth implant systems purchased in 2026 include a 3-year comprehensive warranty covering parts, labor, and onboard electronics. Software updates (including AI-driven treatment planning modules and firmware patches) are included for the first 36 months. Extended warranties with predictive maintenance analytics are available at 15–20% of MSRP per year. Note: Consumables (e.g., burs, sleeves, drill guides) and damage from improper sterilization are excluded. Warranty validation requires quarterly service logs and adherence to OEM maintenance schedules. |
| 5. How are firmware and software upgrades managed post-installation, and are they compatible with evolving implant protocols? | Modern implant platforms use secure OTA (Over-The-Air) update protocols compliant with HIPAA and GDPR. Firmware upgrades are automatically pushed quarterly, with critical security patches deployed within 72 hours of release. All 2026-certified systems support ISO 14971 risk management standards and are backward-compatible with legacy implant databases. Cloud-connected systems now feature adaptive learning algorithms that align with updated prosthetic workflows and zygomatic/pterygoid protocol expansions. Distributors must provide upgrade compliance documentation during annual audits. |
Need a Quote for Price Full Mouth Dental Implants?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


