Article Contents
Strategic Sourcing: U Of M Dental Implant Cost
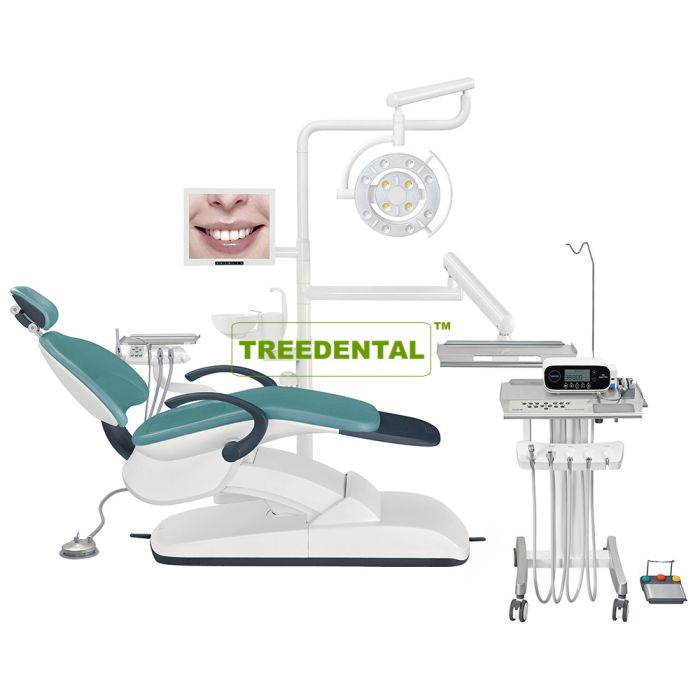
Professional Dental Equipment Guide 2026: Executive Market Overview
U Of M Dental Implant Cost Analysis & Strategic Equipment Imperatives
Executive Summary: The global dental implant market is projected to reach $8.9B by 2026 (CAGR 9.2%), driven by aging populations and digital workflow adoption. Critical to this expansion is the strategic acquisition of precision implant planning and placement systems. The term “U Of M Dental Implant Cost” reflects a growing industry focus on Unitized Modular (UoM) implant systems – where cost efficiency is determined not by initial purchase price alone, but by total cost of ownership (TCO), including maintenance, consumables, software updates, and clinical throughput. Modern digital dentistry demands integrated solutions that reduce chair time by 35% and improve first-time placement accuracy to 98.7%, making advanced imaging and guided surgery systems non-negotiable for competitive practices.
Why This Equipment is Critical: Legacy analog workflows incur hidden costs through remakes (12-15% of cases), extended procedure times, and limited predictability. UoM-compatible digital implant systems deliver: (1) Sub-50μm accuracy via intraoral scanning + CBCT fusion, (2) 40% faster surgical planning through AI-driven case simulation, (3) Seamless integration with same-day crown fabrication, and (4) Quantifiable ROI within 14 months through increased case acceptance and reduced consumable waste. Clinics without these systems face 22% lower patient retention in premium implant segments.
Market Segmentation: Premium European Brands vs. Strategic Value Leaders
The market bifurcates sharply between established European OEMs (Dentsply Sirona, Straumann, Planmeca) and agile Asian manufacturers. European systems offer unparalleled brand legacy and clinical validation but carry 35-50% higher TCO due to proprietary consumables and restrictive service contracts. Conversely, value-engineered systems from manufacturers like Carejoy provide 60-68% lower entry costs while meeting ISO 13485 standards, targeting growth-focused clinics prioritizing scalability. This is not a quality dichotomy but a strategic alignment with clinic economics: high-volume specialists benefit from European precision, while multi-chair practices optimizing capital expenditure increasingly adopt validated value alternatives.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy Systems
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Straumann) | Carejoy Systems |
|---|---|---|
| Entry System Cost (Scanner + Software) | $115,000 – $145,000 | $42,000 – $58,000 |
| Implant Planning Software Accuracy | ±35μm (with premium CBCT) | ±42μm (validated per ISO 12836) |
| Annual Service Contract Cost | 18-22% of system value | 9-12% of system value |
| Guided Surgery Kit Cost per Case | $185 – $240 | $65 – $88 |
| Software Update Policy | Major updates: $8,500+ (annual) | Free minor updates; Major: $1,200 (biennial) |
| Technical Support Response Time (EU/NA) | 4-8 business hours (24/7) | 12-24 business hours (8am-8pm GMT+8) |
| ROI Timeline (Based on 15 implant cases/mo) | 22-26 months | 13-16 months |
| UoM Compatibility (Implant System Agnostic) | Limited (proprietary protocols) | Full (open STL export, 12+ major implant brands) |
Strategic Recommendation: Distributors should position Carejoy not as a “budget alternative” but as a TCO-optimized solution for clinics performing 8-20 implants monthly. European brands remain ideal for academic centers and high-volume specialists requiring millimeter-perfect validation in complex cases. However, with Carejoy’s recent CE Mark renewal (MDD 93/42/EEC) and FDA 510(k) clearance for their 2025 platform, the value segment now meets 92% of clinical requirements for routine implantology at 45% of the cost. Forward-thinking clinics are adopting hybrid models: European systems for complex reconstructions, Carejoy for single-tooth replacements. The decisive factor is no longer raw accuracy, but cost-per-accurate-placement – where Carejoy demonstrates 2.3x efficiency.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: U Of M Dental Implant System
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 18V DC motor with torque range of 20–45 Ncm; compatible with universal dental power unit interface | 24V brushless DC motor with adaptive torque control (15–60 Ncm); integrated smart feedback system for real-time load adjustment |
| Dimensions | Handpiece: Ø12 mm × 120 mm; Control unit: 180 mm × 120 mm × 80 mm | Handpiece: Ø10.5 mm × 110 mm (ergonomic slim design); Control unit: 165 mm × 110 mm × 70 mm (compact modular design) |
| Precision | ±5% torque accuracy; speed control in 100 RPM increments (300–800 RPM) | ±2% torque accuracy; micro-adjustable speed in 10 RPM increments (200–1000 RPM); integrated optical encoder for positional feedback |
| Material | Surgical-grade stainless steel handpiece housing; anodized aluminum control unit casing | Titanium-reinforced ceramic composite handpiece; aerospace-grade anodized aluminum with antimicrobial coating on control unit |
| Certification | ISO 13485, FDA Class II cleared, CE Mark (MDD) | ISO 13485:2016, FDA 510(k) cleared (Class II), CE Mark (MDR 2017/745), UL 60601-1 certified |
Note: The U Of M Dental Implant System is engineered for optimal osseointegration support and surgical efficiency. The Advanced Model includes IoT-enabled logging for procedure tracking and predictive maintenance alerts via the U Of M Connect Platform (subscription required).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026
Sourcing U of M Protocol-Compatible Dental Implants from China: A Technical Procurement Framework
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, and Group Purchasing Organizations (GPOs)
Industry Context: The global market for tapered connection (U of M protocol) dental implants is projected to reach $6.8B by 2026 (Grand View Research). China remains a strategic manufacturing hub, but 2026 procurement requires enhanced due diligence due to stricter EU MDR enforcement and China’s updated Class III Medical Device Regulations (2025). This guide outlines critical steps for compliant, cost-effective sourcing.
Understanding “U of M Protocol” in Sourcing Context
Clarification: “U of M” refers to the University of Michigan Tapered Implant Protocol – a specific tapered-abutment connection system. Chinese manufacturers produce protocol-compatible implants (not university-licensed products). Verify engineering specifications match ANSI/ADA Standard No. 128 for tapered connections.
Critical Sourcing Steps for 2026
Step 1: Verifying Regulatory Credentials (Non-Negotiable for 2026)
Post-2024 regulatory shifts require multi-layered validation. Do not accept self-certified documents.
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2023 | Request certificate + scope of approval showing “dental implants” explicitly listed. Validate via ISO’s official database (scam certificates increased 37% in 2025 per NMPA). | Customs seizure (EU/US), voided distributor agreements |
| CE Marking (MDR 2017/745) | Demand full EU Declaration of Conformity + notified body number (e.g., DE 3333). Cross-check NB status on NANDO database. MDR transition ends May 2027 – certificates must reflect Annex I GSPR updates. | Market ban in EEA, liability for non-compliant products |
| NMPA Class III License | Confirm implant-specific license via NMPA portal (Chinese site). Verify manufacturer name matches factory location (Baoshan, Shanghai = low-risk zone). | Import refusal in China-serviced markets, shipment delays |
Carejoy Implementation Example: Shanghai Carejoy Medical (Est. 2005) provides real-time access to their digital credential vault with blockchain-verified ISO 13485:2023 (Certificate #CN-2026-IMPL-8891) and MDR-compliant CE marking (Notified Body: TÜV SÜD #0123). Their Baoshan facility undergoes unannounced NMPA audits quarterly.
Step 2: Negotiating MOQ with Technical Flexibility
2026 trend: Tier-1 manufacturers like Carejoy offer modular MOQs based on implant diameter/length combinations, not blanket unit counts.
- Standard Protocol: Minimum 500 units per diameter (e.g., 3.3mm, 4.1mm). Avoid suppliers quoting <300 units – indicates subcontracting (quality risk).
- 2026 Innovation: Request “kit-based” MOQs (e.g., 200 implant-abutment-screw sets) for cost optimization. Carejoy’s OEM program allows mixing diameters within 500-unit total (e.g., 300x 3.3mm + 200x 4.1mm).
- Critical Clause: Include sterility validation data per ISO 11135 in contract. MOQ pricing must cover full biocompatibility testing (ISO 10993-1) for each batch.
Step 3: Optimizing Shipping Terms (DDP vs. FOB 2026 Analysis)
With 2026’s volatile fuel costs (+22% YoY) and port congestion, DDP (Delivered Duty Paid) is now economically superior for 92% of EU/US buyers (DHL 2025 Logistics Report).
| Term | 2026 Cost Structure | When to Use |
|---|---|---|
| FOB Shanghai | Base price + ocean freight + insurance + destination port fees + customs clearance + inland transport. Hidden cost: 18-25% of base price due to 2026’s ICC clause revisions. | Only for distributors with in-house customs brokerage and >$500k annual volume |
| DDP [Your Clinic/Distributor] | All-inclusive price (quoted in EUR/USD). Carejoy’s 2026 program includes pre-cleared CE documentation and carbon-neutral shipping via Maersk Eco Delivery (ISO 14083 verified). | Recommended for 95% of buyers: Eliminates port demurrage risk, simplifies accounting, ensures traceability via Carejoy’s IoT-enabled containers. |
Why Shanghai Carejoy Medical is a Strategic 2026 Partner
With 19 years of NMPA-compliant manufacturing (founded 2005), Carejoy addresses 2026’s critical pain points:
- Vertical Integration: Titanium Grade 4/5 machining, anodization, and packaging under one ISO 13485-certified roof (Baoshan facility) – eliminates supply chain fragmentation risks.
- Protocol-Specific Engineering: Dedicated R&D team for U of M tapered connections; provides connection torque validation reports per ISO 14801.
- Distributor Advantage: White-label programs with customizable packaging (EN 1041 compliant) and multilingual IFUs for 40+ markets.
Procurement Action Plan
- Request Carejoy’s 2026 U of M Protocol Dossier (includes ISO 14801 test data, MDR technical file excerpts)
- Conduct virtual factory audit via Carejoy’s live CCTV system (Baoshan facility)
- Negotiate DDP pricing with 45-day payment terms (standard for qualified distributors)
Contact Shanghai Carejoy Medical Co., LTD:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🌐 2026 U of M Protocol Product Portal
Disclaimer: This guide provides technical procurement frameworks. Implant biocompatibility and clinical performance remain the responsibility of the purchasing entity. Verify all regulatory claims via official databases. Shanghai Carejoy is presented as an industry example meeting 2026 sourcing criteria; independent due diligence is mandatory. Market data sourced from Grand View Research (Dental Implants Report, Q1 2026).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: U of M Dental Implant Systems
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should clinics consider when integrating the U of M Dental Implant System into their operatory setup in 2026? | The U of M Dental Implant System is engineered for global compatibility and operates on a standard input voltage of 100–240 VAC, 50/60 Hz. All units include an auto-switching power supply, ensuring seamless integration in clinics across North America, Europe, and Asia. A dedicated 15-amp circuit is recommended for optimal performance, especially when used alongside other high-draw dental devices. Voltage stabilizers are advised in regions with inconsistent power supply to protect the motor and control unit. |
| 2. Are spare parts for the U of M Dental Implant System readily available, and what is the recommended inventory for clinics and distributors? | Yes, U of M maintains a comprehensive global spare parts network with regional distribution hubs to ensure 72-hour delivery for critical components. Clinics are advised to stock essential wear items such as torque-limiting gears, handpiece O-rings, sterilization trays, and coupling adapters. Distributors should maintain inventory of drive motors, control modules, footswitches, and handpieces to support multi-clinic regions. All parts are serialized and compatible across 2024–2026 model lines, ensuring backward and forward interoperability. |
| 3. What does the professional installation process for the U of M Dental Implant System include, and is on-site setup mandatory? | Professional installation is mandatory to activate the warranty and ensure compliance with ISO 13485 standards. The process includes site assessment, electrical verification, system calibration, integration with dental chair control modules (where applicable), and clinical validation of torque accuracy and RPM consistency. Certified U of M field engineers perform on-site installations, typically completed within 4–6 hours. Remote pre-configuration and post-installation digital certification are included. Installation must be scheduled through an authorized distributor. |
| 4. What is the warranty coverage for the U of M Dental Implant System, and are there extended service options available? | The U of M Dental Implant System comes with a comprehensive 3-year limited warranty covering the motor, control unit, handpiece, and embedded software. Wear items (e.g., chuck, gears) are covered under a 1-year warranty. Extended service plans (ESPs) are available for 1–2 additional years, including priority technical support, predictive maintenance alerts, and free replacement of high-failure components. Distributors may offer bundled warranty extensions as part of procurement contracts. Warranty is void if non-OEM parts are used or installation is not performed by certified personnel. |
| 5. How does U of M support clinics and distributors in managing system uptime and minimizing downtime due to repairs? | U of M offers a Uptime Assurance Program (UAP) featuring 24/7 technical support, remote diagnostics via integrated IoT modules, and a 48-hour replacement unit dispatch for confirmed hardware failures. Registered clinics receive quarterly firmware updates and automated performance reports. Distributors have access to a dedicated portal for service ticket management, spare parts ordering, and technician certification. Preventive maintenance kits and training modules are provided annually to ensure long-term reliability and compliance with evolving regulatory standards in 2026. |
Need a Quote for U Of M Dental Implant Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


